Over 2,000 years ago, Hippocrates famously proclaimed,
"All disease starts in the gut."
In 400 BC, he also said,
"Bad digestion is the root of all evil."
"Death sits in the bowels."
In this series we will be discussing the common causes of leaky gut and how to break the cycle of inflammation, keep your organs and body vital, and maximize your healthspan. Understanding the structure of the gut barrier can also help with precisely formulating medical approaches to leaky gut. Through this process, you’ll learn about nutrients, nutraceuticals, and interventions that can get to the root of inflammation and help repair the gut barrier.

Outline:
What exactly is “Leaky Gut” or Intestinal Hyperpermeability?
The function of the gut is to absorb nutrients, ions, and beneficial molecules. The gut is made up of a one-cell-thick layer of intestinal epithelial cells. These epithelial cells are connected by tight junctions, which are proteins that help the cells adhere together and vet which molecules can pass through and get absorbed into the bloodstream.[1]

Normally, tight junction proteins act like a fence, keeping the contents of the gut "out" and the body's internal milieu "in." However, some environmental factors like toxins or chemicals can break the fence. This allows the contents of the gut to enter circulation. Once foreign molecules enter the bloodstream, they can potentially access any organ and trigger systemic inflammation.
Healthy functioning of the gut lining is crucial for a well-adapted body—from digestion and absorption to immune function and brain health.
Layers of the Gut Barrier: Anatomy Overview
Mechanisms of leakage: For gut contents to “leak” outside the gut wall into the body, they need to pass through three major layers: the mucus, the intestinal epithelial cell (IEC) layer, and the underlying immune tissue.[2]
The lumen is the space occupied by food contents and microbes. There is a certain level of defense against leaky gut here. In the lumen, bacteria and antigens are degraded by bile, gastric acid, and pancreatic juice. In addition, commensal bacteria inhibit pathogen colonization by producing antimicrobial substances.
Mucosal Layer
The airways, mouth, digestive tract, genitourinary tract, and skin are lined by a mucus layer full of antimicrobial compounds that prevent bacterial adhesion.[3] The gut mucus layer lays beneath the lumen, and it helps with digestion, nutrient absorption, waste secretion, and keeping the gut relatively alkaline.[4]
The small intestines digest food and absorb nutrients, while the large intestines absorb water and electrolytes. Bacterial density is very high in the large intestines, necessitating a thick inner mucus layer full of antimicrobial compounds and immunoglobulins. This layer is normally impenetrable to bacteria. Compounds in the mucus, like mucins, prevent large particles, including most bacteria, from directly contacting the epithelial cell layer.
Secretory IgA also contributes to barrier function. IgA is made by plasma cells in the lamina propria and is then secreted by B cells into the lumen in collaboration with enterocytes, or intestinal epithelial cells (IECs).[5] IgA binds to specific bacteria, bacterial products, invading microorganisms, and toxins and entraps them in a mucus layer, neutralizing them.[2] IgA also helps goblet cells present bacterial antigens to dendritic cells.[6]
Over time the thick mucus layer turns over, just like skin, and it thins out. This thinner mucus can serve as a “nest” for the microbes, while our IECs are continually working to produce thick mucus near the border.
Intestinal Epithelial Cell Layer
IECs comprise the single-cell thick internal lining of the gut.[6] This layer is composed of different types of specialized intestinal epithelial cells.[7] These include absorptive enterocytes, goblet cells, Tuft cells, enteroendocrine cells, Paneth cells, and microfold cells or M cells.[6]
IECs are rapidly renewed and replaced every couple days. These cells work to maintain barrier integrity. IECs form a brush border at their apical surface to keep things moving. Many intestinal epithelial cells adhering together form villi and crypts, like mountains and valleys. Pore size is relatively smaller at the villus tip and larger at the base of the crypt.[2] As a result, size selectivity varies—the permeability is higher in the crypt and lower at the villus.[3]
IECs also serve immune functions. IECs can react to noxious stimuli by secreting chloride and antimicrobial peptides such as β-defensins and Reg3.[2] They recognize prokaryotic-associated molecular patterns (PAMPs) with toll-like receptors (TLRs) on their surface and nucleotide-binding oligomerization domain (NOD)-like receptors in their cytoplasm, which activate defense mechanisms by secretion of anti-microbial peptides.[6] IECs can also phagocytose bacteria and sequester and neutralize bacteria and toxins, and they communicate with underlying immune cells to regulate inflammatory response against bacterial toxins.
Absorptive enterocytes
Absorptive enterocytes are the most abundant epithelial cells. As their name suggests, they absorb nutrients.
Goblet cells
Goblet cells secrete mucins, among which mucin-2 is the most abundant, which prevents bacterial adhesion to epithelial cells. Goblet cells also secrete molecules that are central to both defense and repair of the epithelial layer, including IgA, mucus, trefoil peptides, and resistin-like molecule-β.[8] Goblet cells depend on commensal bacterial signals to regulate mucus production—germ-free mice have reduced mucus layer thickness; this can be reversed with TLR ligands.[5] Goblet cells can also deliver antigens to the lamina propria.[5] Goblet cells have been reviewed in detail elsewhere.[9]

Tuft cells
Tuft cells are more rare and less well-characterized, but they are essential for immunity against parasitic helminths. In response to helminth infection, these cells orchestrate the type 2 immune response by producing IL-25, which activates innate lymphoid cell type 2 (ILC2) cells. ILC2 cells can in turn recruit eosinophils to protect the intestinal epithelium.[10]
Enteroendocrine cells
Enteroendocrine cells produce and release hormones into the submucosal space to interact with neurons or immune cells. These hormones include substance P, bradykinin, prostanoids, and, in response to various mechanical, chemical, and nervous stimuli, most of the serotonin in the gut.[11] Serotonin stimulates motility and secretion in response to most injurious substances to move them down towards the colon. It also secretes glucagon-like peptide 2 (GLP-2) that is trophic to small bowel epithelium and induces healing.[2]
Paneth cells
Paneth cells are numerous in crypts and establish a chemical barrier by secreting antimicrobial peptides. When exposed to bacteria or bacterial products such as LPS, they produce antimicrobial peptides, including lectins, alpha-defensins, Reg3 proteins (C-type lectins, which target Gram-positive bacterial cell wall peptidoglycans), resistins, cathelicidins, and lysozyme, which disrupt bacterial cell membranes and keep microbes at bay.[2, 5, 6] Paneth cell functions have been reviewed in detail elsewhere.[12, 13]

Microfold cells
Microfold cells (M cells) are specialized antigen-presenting IECs that are part of mucosa-associated lymphoid tissue, or MALT. They comprise around 5% of the follicle-associated epithelium (FAE) and are found above Peyer’s patches and isolated lymphoid follicles (nodes).[5] Microfold cells get their names because they have little folds (sparse, short microvilli) in their membrane that allow them to take up antigens.[14] Critically, M cells secrete IgA, which helps present bacterial antigens in the gut lumen to dendritic cells and to lamina propria. M cells can take up antigens nonspecifically or transport them via receptors. Antigens can bind to receptors on M cells and get internalized into vesicles, where they are transported through transcytosis to underlying lymphoid tissues; from there, they are retained and destroyed.[3, 5] Some microbes actually exploit M cells to infiltrate—including Salmonella typhi, Brucella, Shigella flexneri, botulinum toxin, norovirus, human influenza virus, and prions. M cell-deficient mice have smaller B cell follicles and lower levels of IgA production.[15]
Gut-Associated Lymphoid Tissue (GALT)
GALT is made up of the muscular mucosa (what the whole GALT sits on), Peyer’s patches, lymph nodes, and lamina propria.

Peyer’s patches (PPs) are the most important sites for induction of T cell-dependent IgA class switch recombination. Human young adults have over 200 PPs in their small intestine. They are connected to the mesenteric lymph nodes by afferent lymphatics; they lack lymphatic drainage vessels.[14]
Lamina propria contains innate and adaptive (acquired) immune cells, particularly dendritic cells, plasma cells, macrophages, lymphocytes, and in some cases, neutrophils.[3] These immune cells secrete chemical substances including IgA, cytokines, chemokines, and mast cell proteases. Plasma cells secrete secretory IgA (sIgA) into the gut lumen. B lymphocytes secrete cytokines, chemokines, and anti-inflammatory IL-22. Mast cells secretes proteases, which can mobilize inflammatory responses and clearance of bacterial pathogens.[2]
Dendritic cells (DCs) sample the gut lumen by extending dendritic processes across tight junctions in the distal small intestine.[3] DCs can be conditioned by TGF-β and retinoic acid – both of which can enhance Treg cell differentiation.

Immune tissue also includes intraepithelial lymphocytes within the intestinal epithelial cell layer near the basal side. They are located above basement membrane, but subjacent to tight junction.[3] M cells present antigens to these cells.
CD4+ T cell subtypes
Minor barrier defects allow bacterial products and dietary antigens to cross the epithelium and enter the lamina propria. This can lead to disease or homeostasis. If the foreign materials are taken up by antigen-presenting cells (APCs), such as dendritic cells, that direct the differentiation of T helper 1 (Th1) or Th2 cells, disease can develop. In this process, APCs and Th1 cells can release tumor necrosis factor (TNF) and interferon-gamma (IFN-γ), which signal to epithelial cells to increase flux across the tight junction leak pathway, thereby allowing further leakage of bacterial products and dietary antigens from the lumen into the lamina propria and amplifying the cycle of inflammation. This may, ultimately, culminate in established disease. Alternatively, interleukin-13 (IL-13) released by Th2 cells increases flux across small cation-selective pores, potentially contributing to ongoing disease. Conversely, homeostasis may dominate if APCs promote regulatory T (Treg) cell differentiation, which can be enhanced by epithelial cell-derived transforming growth factor-β (TGF-β) and retinoic acid. The Treg cells display latency-associated peptide (LAP) on their surfaces and may secrete IL-10 and TGF-β to prevent disease.[3]
Leaky gut causes bacteria to translocate and causes expansion of CD4+ T cells and IL-17A production that limits enteric pathogen invasion.[4]
Enteric nervous system
The final layer is the enteric nervous system. This layer activates endocrine and secretomotor mechanisms and mediates intestinal propulsive motility. It also secretes some transmitters, including serotonin and histamine. Serotonin signaling can help harmful molecules move along so they don’t get absorbed.
As you can see, the intestinal epithelial cells are situated between the mucus and the underlying immune system, and they play a major role in gut barrier integrity. Now let’s circle around back to intestinal epithelial cells and take a closer look at how molecules are transported and what goes wrong in leaky gut.
Types of transport in intestinal epithelial cells
Molecules can cross between cells (paracellular transport) or through cells (transcellular transport).[7] Transcellular pathways can involve passive diffusion across the cell membrane or can be carrier or receptor-mediated. Meanwhile, the paracellular pathway involves intramembrane passive diffusion between spaces through adjacent cells, allowing ions and solutes through but preventing proteins, lipids, and microbial-derived peptides from passing through.
Transcellular Pathways
Transcellular transport allows the passage of bacterial / food antigens across cell membrane. There are a few basic kinds: (1) passive transport, (2) passive diffusion by efflux pumps, (3) active transport, and (4) endocytosis.
In passive transport, nonpolar compounds like soluble lipids pass through the phospholipid bilayer by simple diffusion.
Through passive diffusion by efflux pumps, small hydrophilic compounds and nutrients mainly use membrane channels and transporters.
Active transport is carrier-mediated and involves binding of a target molecule to the receptor of the transporter. The carrier undergoes a conformational change and the compound translocates.
Finally, endocytosis[7] occurs for larger molecules, such as proteins and bacterial products. Proteins or peptides bind to specific cell surface receptors, resulting in transcytosis. Endocytosis functions in antigen surveillance in the GI tract. One particular type of endocytosis called phagocytosis involves TLR-mediated internalization of Gram-negative bacteria, viruses, and particles in enterocytes. Next, micropinocytosis internalizes extracellular fluids, dissolved molecules, viruses, and apoptotic cell fragments. Micropinocytosis can be clathrin- or caveolae-mediated. Immunoglobulins and viruses undergo clathrin-mediated endocytosis. Lipid rafts or caveolae mediate internalization of some enterotoxins and viruses into enterocytes. This involves invagination of cholesterol-rich areas of plasma membrane that contain a coating protein called caveolin.
Paracellular Pathways
In the paracellular pathways, there are three basic options: transport, leakage, or apoptosis (unregulated permeability).
The leak pathway allows paracellular transport of large solutes, including limited flux of proteins and bacterial LPS. The size limit hasn’t been defined precisely, but research indicates that whole bacteria are too large to pass. There is no charge selectivity for this pathway.[3] TNF-induced myosin light chain kinase (MLCK) activation seems to increase paracellular flux through the leak pathway.[3]
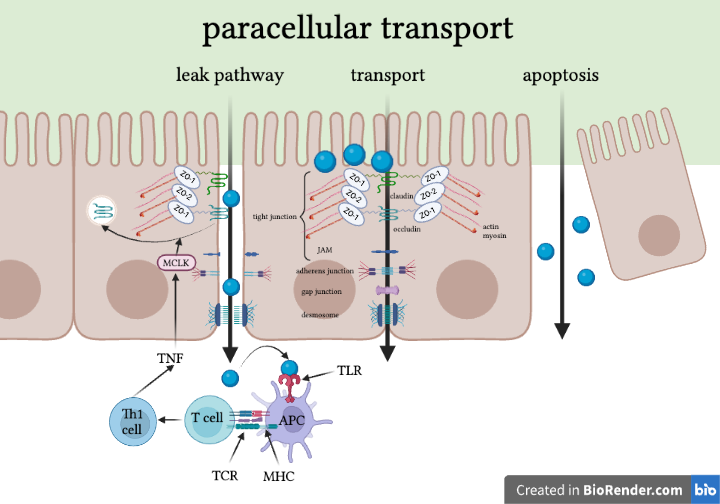
Through formal transport, tight junction-associated claudins define small pores that are the primary determinants of charge selectivity. They exclude molecules larger than 4 angstroms. Expression of claudins can vary by organ (e.g., brain vs. gut) and by region within organ and is modified by external stimuli like cytokines.
Finally, there’s the apoptotic pathway. This pathway is completely unregulated. Extensive apoptosis of epithelial cells is a problem. If cells get extruded, whether apoptotic or alive, intestinal inflammation could ensue if a void is left behind.[5] However, the relevance of single-cell apoptosis to barrier dysfunction remains controversial, as different results are obtained in diverse experimental systems.[3] Numerous stem cells inhabit the crypts of the intestines and quickly proliferate and replenish these holes.
Paracellular transport is mainly what we’re talking about when we’re talking about leaky gut. So let’s take a closer look at that paracellular pathway and which molecules are transported.
Anatomy of the paracellular pathway
Under physiological conditions, only water and solutes like electrolytes can cross the epithelium through the paracellular pathway.[7] The junctional complex of IECs involved in the paracellular pathway includes various proteins. Broadly, the paracellular pathway is composed of the apical junctional complex (which includes tight junctions and adherens junctions), gap junctions, and desmosomes.
Tight junctions are the principal determinant of mucosal permeability. They promote the structural integrity of epithelial cells lining the gut (IECs) and seal the paracellular spaces between epithelial cells.[2] Tight junctions are composed of transmembrane proteins like claudins, occludin, junctional adhesion molecule-A (JAM-A), and intracellular scaffolding proteins, such as zonula occludens (ZO). ZOs act as scaffold proteins, connecting claudins and occludin to cytoskeletal actin.[7] Finally, cytoplasmic proteins help coordinate cytoskeletal (actin and myosin) dynamics.[4, 5] Occludin endocytosis is a signal of leakage.
Tight junctions limit the transport of hydrophilic molecules. They regulate size and charge selectivity and allow some medium-sized hydrophilic molecules, ions, or positively charged molecules to pass through. They prevent transport of proteins, such as antigenic macromolecules, lipids, and microbial-derived peptides. They are responsible for water, certain small molecules, and ions passing through the barrier.[4] NHE3 (apical sodium-hydrogen exchange protein 3 is required for transcellular Na+ absorption in the intestines and kidneys; it also contributes to the sodium gradient that drives paracellular water absorption.[3] The tight junction limits solute flux along paracellular pathway, which is typically more permeable than transcellular pathway. Overall, the tight junction is charge and size-selective and is the rate-limiting step in transepithelial transport.[3]
Adherens junction plays a role in the stabilization of cell-cell contacts. Finally, desmosomes are located under the apical junctional complex and join adjacent cells together and provide anchoring sites for intermediate filaments.[4]
Key Takeaways
The major layers of the gut include the lumen, the mucus, the intestinal epithelial cell (IEC) layer, and the underlying gut-associated lymphoid tissue (GALT), the enteric nervous system, and muscle.
The IEC layer consists of absorptive enterocytes, goblet cells, Tuft cells, enteroendocrine cells, Paneth cells, and M cells. Goblet cells secrete mucus, while Paneth cells secrete antimicrobial compounds to keep microbes from getting too close to the gut barrier. Tuft cells and M cells have immune functions within the IEC layer. Enteroendocrine cells release hormones including serotonin and signal to the enteric nervous system to stimulate smooth muscle contractions.
The underlying immune tissue consists of the lamina propria, Peyer’s patches, and lymph nodes sitting on the muscular mucosa.
CD4+ T cells include Th1, Th2, Th17, and Treg cells. Th1, Th2, and Th17 cells assist in defense against pathogens, Th2 play a role in allergies, and Tregs have anti-inflammatory effects.
Food or microbial products can be transported transcellularly (across the cells) or paracellularly (between cells). Paracellular transport is what we’re talking about when we refer to leaky gut.
Paracellular anatomy consists of tight junctions, adherens junctions, gap junctions, and desmosomes. Tight junctions are the primary regulators of leaky gut.
References
Nagpal, R. and H. Yadav, Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann Nutr Metab, 2017. 71 Suppl 1: p. 11-16.
Camilleri, M., What is the leaky gut? Clinical considerations in humans. Curr Opin Clin Nutr Metab Care, 2021. 24(5): p. 473-482.
Turner, J.R., Intestinal mucosal barrier function in health and disease. Nat Rev Immunol, 2009. 9(11): p. 799-809.
Inczefi, O., et al., The Influence of Nutrition on Intestinal Permeability and the Microbiome in Health and Disease. Front Nutr, 2022. 9: p. 718710.
Peterson, L.W. and D. Artis, Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol, 2014. 14(3): p. 141-53.
Chelakkot, C., J. Ghim, and S.H. Ryu, Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med, 2018. 50(8): p. 1-9.
Barbara, G., et al., Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front Nutr, 2021. 8: p. 718356.
Johansson, M.E., Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis, 2014. 20(11): p. 2124-31.
Gustafsson, J.K. and M.E.V. Johansson, The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol, 2022. 19(12): p. 785-803.
Steele, S.P., S.J. Melchor, and W.A. Petri, Jr., Tuft Cells: New Players in Colitis. Trends Mol Med, 2016. 22(11): p. 921-924.
Read, N.W. and K.A. Gwee, The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol Ther, 1994. 62(1-2): p. 159-73.
Barreto, E.B.L., et al., Paneth cells and their multiple functions. Cell Biol Int, 2022. 46(5): p. 701-710.
Wallaeys, C., N. Garcia-Gonzalez, and C. Libert, Paneth cells as the cornerstones of intestinal and organismal health: a primer. EMBO Mol Med, 2023. 15(2): p. e16427.
Kobayashi, N., et al., The Roles of Peyer's Patches and Microfold Cells in the Gut Immune System: Relevance to Autoimmune Diseases. Front Immunol, 2019. 10: p. 2345.
Zhang, Z., et al., An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm Sin B, 2021. 11(8): p. 2449-2468.


Comments